MAKING CHALLENGING DISEASES CURABLE - FOR EVERYONE
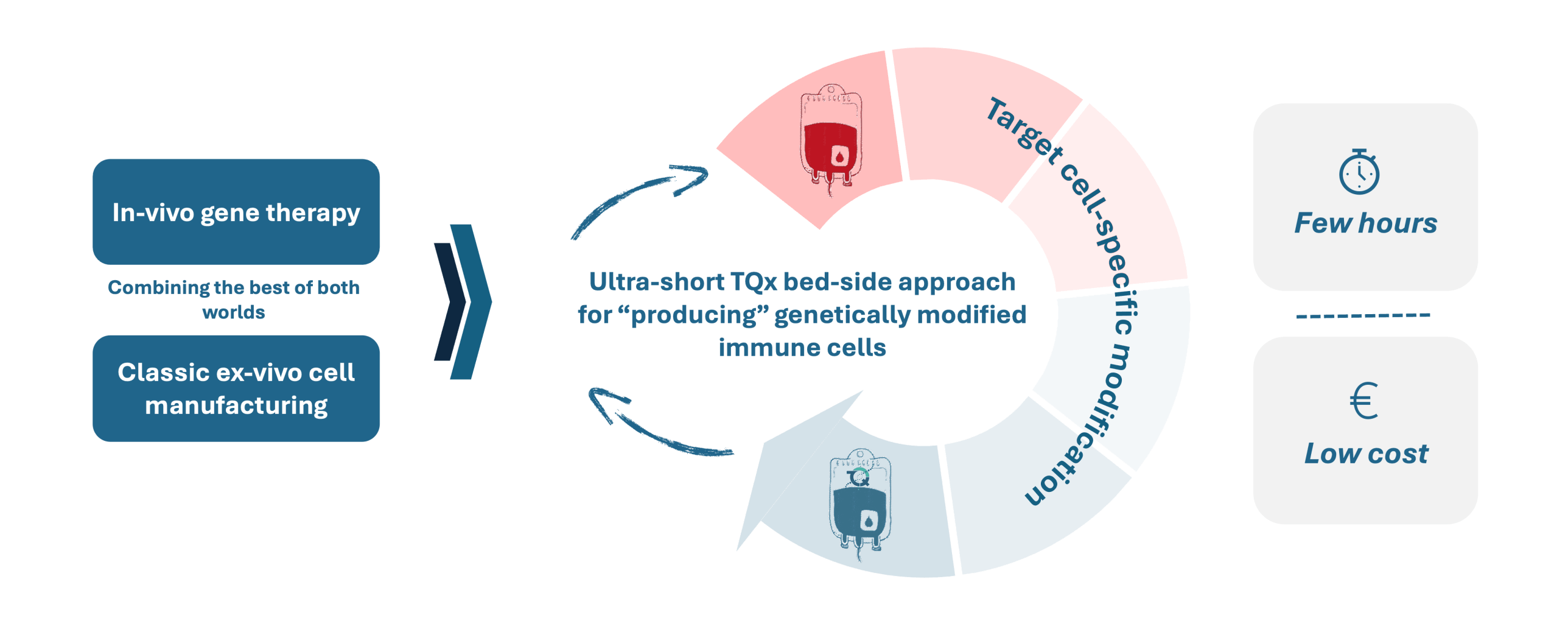
The TQx platform will transform the traditional way of producing cellular therapies, which is often slow, expensive, and hard to scale, into a very fast and highly automated process right at the patient's side. This method uses extra-corporeal gene therapy but keeps the benefits of autologous cell therapy, where patients receive treatments made from their own cells.
Our TQx method makes it easier to create genetically modified immune cells (like CAR-T cells) and deliver cell therapy directly to the patient. This avoids the complicated and costly steps of centralized production.
As a result, this approach unlocks the full potential of autologous cell therapies for treating conditions that were previously difficult to address, including rare autoimmune diseases and cancers.
TQx is developing a modular target cell-specific modification platform including relevant technology components for the generation of gene modified cell therapy products.
- Limited availability for patients
- High burden on patient
- Exhausted cells
- Laborious manufacturing & logistics
- Expensive therapies
- Limited in indications
- Accessibility for all patients
- Superior treatment experience for patients
- Better cell products
- Fast manufacturing and no logistics
- Economic therapies
- Expansion to broader indications
A major problem with current autologous cell therapeutics is their complex production.
Each therapeutic product is manufactured individually for individual patients in special GMP cleanroom facilities - which takes several weeks. On the other hand, generalized in vivo gene therapy is lacking the required high degree of target cell specificity as a prerequisite for the generation of highly effective and safe cellular medicines.
Patients are suffering from an extremely high emotional and physical toll.
TQx is shifting the less scalable, lengthy and cost-intensive "classical" pharmaceutical production of cell therapeutics to a highly automated and ultra-fast gene therapy approach, without abandoning the advantages of autologous cell therapy.
Our ultra-fast process brings the cell therapy directly to the patient instead of producing it under conventional conditions in centralized facilities with the aim of significantly simpler logistics and cost reductions. As a result, the TQx process opens opportunities for the treatment of previously unaddressed indications such as (rare) autoimmune diseases and cancer.
TQx is making cell and gene therapy broadly accessible in order to address the medical needs of many patients.


Andreas has launched several biotech companies, which he managed in leading positions. Among others, he was CEO of Kinaxo, a proteomics service provider that was acquired by Evotec, and NEO New Oncology, a cancer diagnostics company that was acquired by Siemens Healthcare.

Christian obtained his PhD in Cell Biology / Biochemistry at the University of Giessen and moved to the US for his Postdoc position at the Scripps Research Institute in San Diego, CA. Subsequently Christian led projects on the generation, differentiation, and application of human stem cells in various disease models in the drug discovery process in two Startup Companies. In 2018, he moved to Evotec in Hamburg, Germany.

Over the last two decades, Herbert Stadler has built a track record as one of Germany's most active biotech entrepreneurs. He has founded or co founded several (biotech) companies including Biometra GmbH, Ribonetics GmbH, HepaVec AG, IBA GmbH, Stage Cell Therapeutics GmbH and DeveloGen AG.

Emmy Noether group leader & MD. CAR-T cells, cell therapy, immunotherapy, cancer research.

Chief Executive Officer - Mayr BioMedTech Consulting; biopharmaceutical executive; cell & gene therapy & genome editing.
TQ Therapeutics participates in European consortium to decentralize CAR-T cell therapy and improve hospital workflows (Open)
TQ Therapeutics GmbH Announces Acquisition of Juno Therapeutics GmbH (Open)
Pantherna Therapeutics and TQ Therapeutics announce research collaboration to advance ultra-short Cell and Gene Therapy (CGT) process leveraging LNP technology (Open)